New York, USA, Feb. 21, 2025 (GLOBE NEWSWIRE) -- Cell and Gene Therapies in Rare Disorders Market to Skyrocket Across the 7MM During the Forecast Period (2025–2034) | DelveInsight
The cell and gene therapies in rare disorders market is expected to grow at a significantly high rate during the forecast period (2025–2034) and mainly be driven by an increase in approval of a growing number of gene therapies and readily adoption on approval, ability to treat a broad array of conditions, increase in the number of cases, expected one-time dosing approach and curative treatment options.
DelveInsight’s Cell and Gene Therapies in Rare Disorders Market Insights report includes a comprehensive understanding of current treatment practices, emerging cell and gene therapies for various rare disorders, market share of individual therapies, and current and forecasted market size from 2020 to 2034, segmented into 7MM [the United States, the EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan].
Key Takeaways from the Cell and Gene Therapies in Rare Disorders Market Report
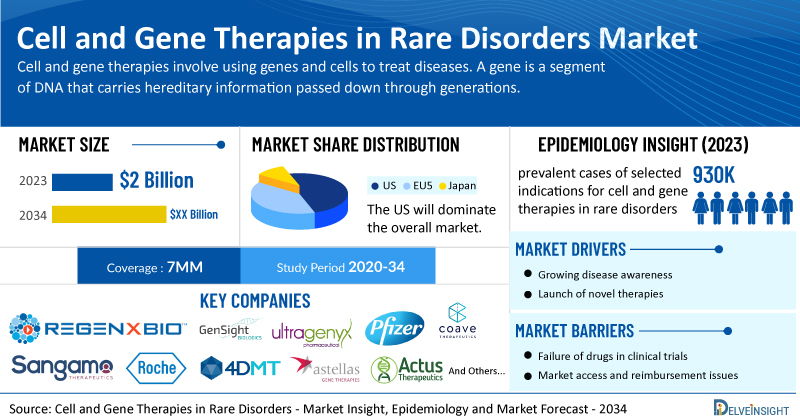
- The total market size in the 7MM for cell and gene therapies in rare disorders was estimated to be nearly USD 2 billion in 2023, which is expected to grow significantly by 2034 as a growing number of companies receive investigational new drug applications each year for these treatments, along with rising regulatory approval in the United States and Europe.
- By 2034, among all the indications, the highest revenue is expected to be generated from Hemophilia A, followed by Retinitis Pigmentosa in the US.
- The total prevalent cases of selected indications for cell and gene therapies in rare disorders in the 7MM comprised approximately 930K in 2023 and are projected to increase during the forecasted period.
- Prominent companies working in the domain of cell and gene therapies in rare disorders, including REGENXBIO, Coave Therapeutics, GenSight Biologics, Ultragenyx, Pfizer, Sangamo Therapeutics, Roche, 4D Molecular Therapeutics, Astellas Gene Therapie, Actus Therapeutics, Nanoscope Therapeutics, Ocugen, jCyte, Amicus Therapeutics, Capricor Therapeutics, Nippon Shinyaku, Brainstorm Cell Therapeutics, Editas Medicine, Abeona Therapeutics, Ishin Pharma, and others, are actively working on innovative cell and gene therapies in rare disorders. These novel cell and gene therapies in rare disorders are anticipated to enter the market in the forecast period and are expected to change the market.
- Some of the emerging cell and gene therapies in rare disorders include RGX-121, Isaralgagene civaparvovec (ST-920), CTx-PDE6b (HORA-PDE6b), GS030, DTX301, Giroctocogene fitelparvovec, Dirloctocogene samoparvovec, 4D-310, AT845, ACTUS-101, MCO-010 (sonpiretigene isteparvovec), OCU400, jCell, AT-GTX-502 (scAAV9.P546.CLN3), CAP-1002, NurOwn (MSC-NTF cells), EDIT-301, EB-101, ISN001, UX111 (ABO-102), and others.
- In December 2024, EyeDNA Therapeutics (subsidiary of Coave Therapeutics) announced it had received Rare Pediatric Disease Designation (RPD) from the FDA for its Investigational Gene Therapy HORA-PDE6b for patients with retinal dystrophy due to PDE6b gene mutations.
- In February 2024, REGENXBIO announced the topline results of RGX-121 for the treatment of Hunter syndrome in which, the pivotal phase of the trial met its primary endpoints with statistical significance.
Discover which therapies are expected to grab the major cell and gene therapies in rare disorders market share @ Cell and Gene Therapies in Rare Disorders Market Report
Cell and Gene Therapies in Rare Disorders Overview
Cell and gene therapies involve using genes and cells to treat diseases. A gene is a segment of DNA that carries hereditary information passed down through generations. All the genes collectively make up the genome, and they can contain information about physical traits, like height or eye color. Many genes provide instructions for producing RNA or protein molecules, which, though not visible, play crucial roles in the body’s cells. Cells are the basic building blocks of living organisms (including humans), acting as functional units that combine to form organs and tissues.
Gene therapy involves using genetic material to address genetic disorders. This can include adding a healthy version of a gene (gene addition) or correcting a mutated gene to its normal form (gene editing). The therapy can be administered either outside the body (ex vivo) or within the body (in vivo). Modified viruses or other delivery methods are used to introduce the gene into the cells' genome. Cell therapy, on the other hand, uses cells from the patient or a donor to treat diseases. Often, stem cells are used in cell therapy because they can develop into specialized cells.
These cells may or may not be genetically modified. In some cases, it's easier to remove cells from the body, treat them with gene therapy, and then reintroduce them, rather than applying the treatment directly within the body. This is common in treatments for blood disorders, where gene therapy and cell therapy are often combined.
Cell and Gene Therapies in Rare Disorders Epidemiology Segmentation
The cell and gene therapies in rare disorders epidemiology section provides insights into the historical and current cell and gene therapies in rare disorders patient pool and forecasted trends for the 7MM. It helps recognize the causes of current and forecasted patient trends by exploring numerous studies and views of key opinion leaders.
The cell and gene therapies in rare disorders market report proffers epidemiological analysis for the study period 2020–2034 in the 7MM segmented into:
- Total Prevalent Cases of Selected Indications for Cell and Gene Therapies in Rare Disorders
- Total Indication-wise Eligible Cases for Cell and Gene Therapies in Rare Disorders
- Total indication-wise Treated Cases for Cell and Gene Therapies in Rare Disorders
Download the report to understand which factors are driving cell and gene therapies in rare disorders epidemiology trends @ Cell and Gene Therapies in Rare Disorders Epidemiological Insights
Cell and Gene Therapies in Rare Disorders Treatment Market
Cell and gene therapies facilitate the regeneration of diseased tissues by introducing healthy or engineered cells that can directly replace damaged tissue, support the recovery of existing healthy cells, or release factors that help repair affected cells. The approval of therapies such as LUXTURNA, HEMGENIX, ZYNTEGLO LIBMELDY, SKYSONA, HOLOCLAR, UPSTAZA, ROCTAVIAN, and others has established regulatory pathways for other cell and gene therapies currently in development.
The landscape of the cell and gene therapy market is expected to evolve as companies worldwide actively work on developing new treatment options for a diverse range of conditions, including hemophilia A and B, lysosomal storage disorders (such as Fabry, Pompe, Danon Disease, and various types of mucopolysaccharidoses), neurological disorders (like Batten disease and Parkinson’s), musculoskeletal conditions (including DMD, myotubular myopathy, and LGMD), and eye diseases (such as achromatopsia, choroideremia, limbal stem cell deficiency, retinitis pigmentosa, retinoschisis, and age-related macular degeneration).
Additionally, research is advancing in areas like diabetic macular edema, metabolic disorders (e.g., Wilson’s disease, phenylketonuria, and urea cycle disorders), dystrophic epidermolysis bullosa, gangliosidosis, and xerostomia. In recent years, the treatment landscape for rare disorders has transformed significantly due to advancements in cell and gene therapies.
Companies specializing in these therapies are now focusing on developing innovative solutions, particularly for rare genetic diseases. However, identifying the right candidates for cell and gene therapy requires thorough clinical evaluation, with long-term follow-up of study participants to ensure safety and efficacy. Overall, the future of cell and gene therapies appears highly promising.
Learn more about the FDA-approved cell and gene therapies for rare disorders @ Approved Cell and Gene Therapies in Rare Disorders Treatment
Emerging Cell and Gene Therapies in Rare Disorders and Key Companies
Some of the drugs in the pipeline include RGX-121 (REGENXBIO), isaralgagene civaparvovec (ST-920; Sangamo Therapeutics), CTx-PDE6b (HORA-PDE6b; Coave Therapeutics), GS030 (GenSight Biologics), DTX301 (Ultragenyx), and others.
RGX-121 is an experimental, one-time gene therapy designed to introduce the iduronate 2-sulfatase (IDS) gene, which encodes the iduronate 2-sulfatase (I2S) enzyme, using the NAV AAV9 vector. The protein expressed by RGX-121 is structurally identical to natural I2S. This therapy is administered directly into the central nervous system (CNS) via intracisternal or intracerebroventricular delivery. By delivering the IDS gene to CNS cells, the therapy aims to establish a lasting source of secreted I2S beyond the blood-brain barrier (BBB), enabling sustained cross-correction of cells throughout the CNS.
Currently, in Phase III clinical trials for treating Hunter syndrome, RGX-121 has received multiple regulatory designations, including Orphan Drug Designation (ODD), Rare Pediatric Disease Designation, Fast Track Designation (FTD), and Regenerative Medicine Advanced Therapy (RMAT) status from the U.S. FDA, as well as Advanced Therapy Medicinal Product (ATMP) classification from the European Medicines Agency (EMA).
Isaralgagene civaparvovec is a liver-targeting rAAV 2/6 vector that carries the cDNA for human α-Gal A and is administered via a single-dose intravenous infusion. Its goal is to introduce a functional copy of the GLA gene into liver cells, enabling them to produce functional α-Gal A. The company is currently conducting the Phase I/II STAAR clinical trial to evaluate its potential for treating Fabry disease.
The therapy has received Fast Track Designation (FTD), Orphan Drug Designation (ODD), and Regenerative Medicine Advanced Therapy (RMAT) status from the US FDA, as well as PRIME eligibility and Orphan Medicinal Product designation from the EMA.
In October 2024, the company announced a successful regulatory interaction with the US FDA, outlining a clear path to Accelerated Approval for isaralgagene civaparvovec (ST-920). The company expects to generate data supporting this pathway in the first half of 2025, with a potential Biologics License Application (BLA) submission planned for the second half of 2025.
CTx-PDE6b is an AAV5-based gene therapy aimed at delivering a full-length, non-mutated copy of the functional human PDE6b gene into the subretinal space. This approach enables rapid and robust transgene expression, leading to the production of functional PDE6b proteins in photoreceptive rods and cones. The company is currently conducting a Phase I/II clinical trial to evaluate its potential in treating Inherited retinal dystrophy. The therapy has also been granted Orphan Drug Designation (ODD) and Rare Pediatric Disease Designation (RPDD) by the US FDA.
The other cell and gene therapies in rare disorders include
- Giroctocogene fitelparvovec: Pfizer/Sangamo Therapeutics
- Dirloctocogene samoparvovec: Roche
- 4D-310: 4D Molecular Therapeutics
- AT845: Astellas Gene Therapie
- ACTUS-101: Actus Therapeutics
- MCO-010 (sonpiretigene isteparvovec): Nanoscope Therapeutics
- OCU400: Ocugen
- jCell: jCyte
- AT-GTX-502 (scAAV9.P546.CLN3): Amicus Therapeutics
- CAP-1002: Capricor Therapeutics/Nippon Shinyaku
- NurOwn (MSC-NTF cells): Brainstorm Cell Therapeutics
- EDIT-301: Editas Medicine
- EB-101: Abeona Therapeutics
- ISN001: Ishin Pharma
- UX111 (ABO-102): Ultragenyx Pharmaceutical, and others
The anticipated launch of these emerging cell and gene therapies are poised to transform the rare disorders market landscape in the coming years. As these cutting-edge therapies continue to mature and gain regulatory approval, they are expected to reshape the rare disorders market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
To know more about cell and gene therapies in rare disorders clinical trials, visit @ Emerging Cell and Gene Therapies in Rare Disorders
Cell and Gene Therapies in Rare Disorders Market Dynamics
The cell and gene therapies in rare disorders market dynamics are anticipated to change in the coming years. AAVs have emerged as the predominant vectors for delivering genes of interest to target tissues with improved specificity, efficiency, and safety, making them increasingly relevant for rare disease therapies. Companies in this space often benefit from niche markets, lower competition, and higher pricing power.
To encourage the development of treatments for rare diseases, including gene therapies, biopharmaceutical companies are incentivized by Orphan and Fast Track Designations, as well as longer market exclusivity. Gene therapies hold the potential for a paradigm shift in rare disease treatment, moving from symptomatic alleviation to disease modification and even cure.
Furthermore, many potential cell and gene therapies are being investigated for the treatment of rare disorders, and it is safe to predict that the treatment space will significantly impact the market during the forecast period. Moreover, the anticipated introduction of emerging therapies with improved efficacy and a further improvement in the diagnosis rate is expected to drive the growth of the cell and gene therapies in rare disorders market in the 7MM.
However, several factors may impede the growth of the cell and gene therapies in rare disorders market. Despite advancements since the passage of the Orphan Drug Act, US patients with rare diseases still face significant barriers to diagnosis, care, and treatment. Even when therapies are available, the high out-of-pocket costs can be prohibitive. Orphan drugs are often priced higher than common treatments and generics to generate sufficient returns on investment, given small patient populations.
As more companies enter the gene therapy market, production capacity may become more competitive, potentially raising prices or limiting the utility of these treatments. Additionally, challenges like small patient groups, poorly understood disease processes, limited natural history data, and a lack of suitable outcomes hinder the development of effective cell and gene therapies.
Moreover, rare disorders treatment poses a significant economic burden and disrupts patients’ overall well-being and QOL. Furthermore, the cell and gene therapies in rare disorders market growth may be offset by failures and discontinuation of emerging therapies, unaffordable pricing, market access and reimbursement issues, and a shortage of healthcare specialists. In addition, the undiagnosed, unreported cases and the unawareness about the disease may also impact the cell and gene therapies in rare disorders market growth.
| Report Metrics | Details |
| Study Period | 2020–2034 |
| Coverage | 7MM [The United States, the EU-4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan] |
| Cell and Gene Therapies in Rare Disorders Market Size in 2023 | USD 2 Billion |
| Key Cell and Gene Therapies in Rare Disorders Companies | REGENXBIO, Coave Therapeutics, GenSight Biologics, Ultragenyx, Pfizer, Sangamo Therapeutics, Roche, 4D Molecular Therapeutics, Astellas Gene Therapie, Actus Therapeutics, Nanoscope Therapeutics, Ocugen, jCyte, Amicus Therapeutics, Capricor Therapeutics, Nippon Shinyaku, Brainstorm Cell Therapeutics, Editas Medicine, Abeona Therapeutics, Ishin Pharma, and others |
| Key Pipeline Cell and Gene Therapies in Rare Disorders Therapies | RGX-121, Isaralgagene civaparvovec (ST-920), CTx-PDE6b (HORA-PDE6b), GS030, DTX301, Giroctocogene fitelparvovec, Dirloctocogene samoparvovec, 4D-310, AT845, ACTUS-101, MCO-010 (sonpiretigene isteparvovec), OCU400, jCell, AT-GTX-502 (scAAV9.P546.CLN3), CAP-1002, NurOwn (MSC-NTF cells), EDIT-301, EB-101, ISN001, UX111 (ABO-102), and others |
Scope of the Cell and Gene Therapies in Rare Disorders Market Report
- Therapeutic Assessment: Cell and Gene Therapies in Rare Disorders current marketed and emerging therapies
- Cell and Gene Therapies in Rare Disorders Market Dynamics: Conjoint Analysis of Emerging Cell and Gene Therapies in Rare Disorders Drugs
- Competitive Intelligence Analysis: SWOT analysis and Market entry strategies
- Unmet Needs, KOL’s Views, Analyst’s Views, Cell and Gene Therapies in Rare Disorders Market Access and Reimbursement
Discover more about cell and gene therapies for rare disorders in development @ Cell and Gene Therapy Clinical Trials
Table of Contents
| 1 | Cell and Gene Therapies in Rare Disorders Market Key Insights |
| 2 | Cell and Gene Therapies in Rare Disorders Market Report Introduction |
| 3 | Cell and Gene Therapies in Rare Disorders Market Key Highlights from Report |
| 4 | Executive Summary of Cell and Gene Therapies in Rare Disorders |
| 5 | Key Events: Cell and Gene Therapies in Rare Disorders |
| 6 | Cell and Gene Therapies in Rare Disorders Epidemiology and Market Forecast Methodology |
| 7 | Cell and Gene Therapies in Rare Disorders Market Overview at a Glance in the 7MM |
| 8 | Disease Background and Overview of Cell and Gene Therapies in Rare Disorders |
| 9 | Epidemiology and Patient Population |
| 10 | Marketed Cell and Gene Therapies in Rare Disorders |
| 11 | Emerging Cell and Gene Therapies in Rare Disorders |
| 12 | Cell and Gene Therapies in Rare Disorders: 7MM analysis |
| 13 | Unmet Needs |
| 14 | SWOT Analysis |
| 15 | KOL Views |
| 16 | Market Access and Reimbursement |
| 17 | Appendix |
| 18 | DelveInsight Capabilities |
| 19 | Disclaimer |
| 20 | About DelveInsight |
Related Reports
Gene Therapy Competitive Landscape
Gene Therapy Competitive Landscape – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key gene therapy companies, including Novartis, Johnson & Johnson, Fibrocell Technologies, Pfizer, HELIXMITH Co., Ltd., Sarepta Therapeutics, REGENXBIO, Solid Biosciences Inc., Lexeo Therapeutics, Spark Therapeutics, Xalud Therapeutics, uniQure, Ultragenyx Pharmaceutical, Nanoscope Therapeutics, among others.
Cell And Gene Therapy For Multiple Myeloma Market
Cell And Gene Therapy For Multiple Myeloma Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key cell and gene therapy for multiple myeloma companies, including Pfizer, MorphoSys, Biokine Therapeutics, Arcellx, Rapa Therapeutics, among others.
Retinitis Pigmentosa Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key gene therapy In retinitis pigmentosa companies, including Johnson & Johnson Innovative Medicine, MeiraGTx, Beacon Therapeutics, Nanoscope Therapeutics, Gensight Biologics, 4D Molecular Therapeutics, Coave Therapeutics, Ocugen, Bionic Sight, jCyte, Endogena Therapeutics, ProQR Therapeutics, Aldeyra Therapeutics, among others.
Adeno-Associated Virus Vectors in Gene Therapy Market
Adeno-Associated Virus Vectors in Gene Therapy Market Insights, Epidemiology, and Market Forecast – 2032 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key adeno-associated virus vectors in gene therapy companies, including Pfizer, CSL Behring, Spark Therapeutics, Freeline Therapeutics, RegenxBio, Amicus Therapeutics, among others.
Adeno-Associated Virus Vectors in Gene Therapy Pipeline
Adeno-Associated Virus Vectors in Gene Therapy Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key adeno-associated virus vectors in gene therapy companies, including Pfizer, CSL Behring, Spark Therapeutics, Freeline Therapeutics, RegenxBio, Amicus Therapeutics, among others.
DelveInsight’s Pharma Competitive Intelligence Service tailored to rare disease domain: DelveInsight’s competitive intelligence services provide real-time, accurate insights across the different therapeutic domains and rare diseases is one of our fortes, providing detailed insights into advancements across rare disorders such as multiple myeloma, NF1-PN, Graves’ disease, Ewing sarcoma, retinitis pigmentosa, PCPG, etc to name a few. Our services analyze competitors’ pipelines, clinical trial progress, gene and cell therapy innovations, and market entry strategies, as well as regulatory updates and patent landscapes specific to rare diseases. This intelligence enables stakeholders to identify emerging threats, uncover growth opportunities, and develop targeted strategies to remain competitive in this evolving therapeutic area. Connect with us today to explore how we can help you succeed in the rare disease market.
Other Business Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter

Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
